Abstract
BACKGROUND
Pomalidomide (POM; Imnovid) in combination with low-dose dexamethasone (LoDex) demonstrated a significant improvement in progression-free survival (PFS; median, 4.0 vs 1.9 months; P < .0001) and overall survival (OS; median, 12.7 vs 8.1 months; P = .0285) vs high-dose dexamethasone in a phase 3 study of patients with relapsed/refractory multiple myeloma (RRMM; MM-003; San Miguel et al. Lancet Oncol. 2013;14:1055-1066). This trial led to the European approval of POM + LoDEX in patients with RRMM previously treated with ≥ 2 regimens, including lenalidomide (LEN) and bortezomib (BORT), and who had disease progression on their last therapy. However, data on the use of POM in the real-world setting are limited. The goal of the MIROIR study is to investigate the usage, efficacy, and tolerability of POM in current clinical practice in France. Results from a pre-specified 3-year interim analysis are presented.
METHODS
MIROIR is a multicenter, non-interventional study of POM in routine clinical practice. Adults (aged ≥ 18 years) with multiple myeloma who initiated POM treatment in France between October 1, 2014, and September 30, 2017, were included (data cutoff, February 1, 2018). All patients were required to be enrolled in the Imnovid registry (a non-interventional post-marketing authorization registry) and to provide consent. Key exclusion criteria included previous treatment with POM or simultaneous participation in a clinical trial. Patients were followed up for ≤ 24 months after treatment initiation. Data were collected from patient medical files. The primary endpoint is PFS at 6 months. PFS is defined as the time from POM treatment initiation to the first progression according to International Myeloma Working Group criteria or death from any cause. Key secondary endpoints include OS, time to next treatment (TTNT), and safety. This study is ongoing; targeted enrollment is 3000 patients (ClinicalTrials.gov, NCT02902900).
RESULTS
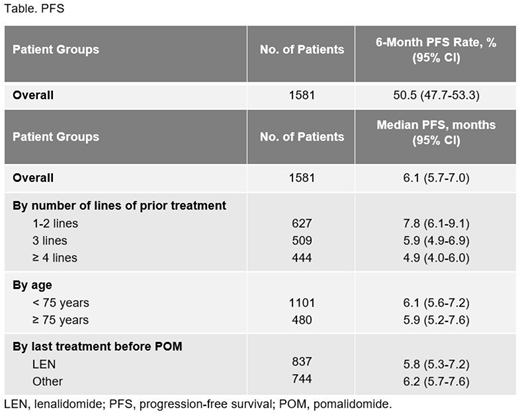
A total of 1581 patients were included in this analysis (median follow-up, 19.1 months). The median age was 69.8 years, and 480 patients (30.4%) were aged ≥ 75 years; 844 patients (53.4%) were male. The median time from first-line treatment to POM initiation was 52.6 months. Patients had received a median of 3 prior lines of therapy (range, 0-9), with 628 (39.7%), 509 (32.2%), and 444 patients (28.1%) receiving ≤ 2, 3, and ≥ 4 prior lines, respectively. The most common prior treatments included BORT (97.3%), LEN (97.0%), melphalan (80.3%), and autologous/allogeneic stem cell transplant (50.0%). POM was prescribed at 4 mg/day in 923 patients (83.8%) aged < 75 years and in 300 patients (62.5%) aged ≥ 75 years. Dexamethasone was prescribed at 20 mg/day and 40 mg/day in 382 (34.7%) and 566 patients (51.4%) aged < 75 years and at 20 mg/day and 40 mg/day in 287 (59.8%) and 49 patients (10.2%) aged ≥ 75 years. The 6-month PFS rate was 50.5% (95% CI, 47.7%-53.3%). Other key PFS data are reported in the Table. Median TTNT was 9.8 months (95% CI, 9.0-10.6 months). The 12-month OS rate was 68.7% (95% CI, 66.1%-71.0%), with a median OS of 22.4 months (95% CI, 21.0-24.6 months). A total of 233 serious adverse events (AEs) related to POM were reported; 26 (11.2%) were neutropenia, 13 (5.6%) were pancytopenia, and 13 (5.6%) were thrombocytopenia. POM dose was reduced due to an AE in 20.0% of patients; treatment was discontinued or interrupted due to an AE in 14.9% and 35.2% of patients, respectively. Many patients received concomitant treatments and supportive care in the form of thromboprophylaxis (81.2%), antiviral prophylaxis with valacyclovir (63.3%), antibiotic prophylaxis (55.8%), bisphosphonates (20.9%), erythropoietin (20.1%), and granulocyte colony-stimulating factor (8.8%).
CONCLUSIONS
The results of this interim analysis of the real-world MIROIR study confirm the efficacy of POM reported in clinical trials and underscore its role as a treatment in RRMM. Median PFS was numerically longer in patients who had received only 1 to 2 prior lines of therapy, supporting earlier initiation of POM. The results also indicate that POM is effective in patients whose disease has relapsed or developed resistance to LEN; PFS outcomes were similar regardless of whether patients had received LEN or another treatment as their most recent therapy. This suggests that there is no need to switch from an immunomodulatory agent to another class of treatment after relapse or resistance to LEN.
Hulin:Celgene: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Macro:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Financial support for congress. Gourgou:Roche: Other: Expertise methodological seminar Force 1 since 2007 and real-life study ; Celgene Corporation: Other: Expertise methodological mirror . Lachenal:Celgene: Other: Scientific Committees . Stoppa:Celgene Corporation: Honoraria. Moreau:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Perrot:Celgene: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Takeda: Honoraria; Sanofi: Honoraria. Mohty:MaaT Pharma: Consultancy, Honoraria. Karlin:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support. Fohrer:Celgene: Consultancy, Honoraria. Sylvain:Gilead: Other: scientific advisor board. Leleu:Celgene: Honoraria, Other: steering committee membership ; Janssen: Honoraria, Other; BMS: Honoraria, Other: steering committee membership ; Merk: Honoraria, Other: steering committee membership ; Takeda: Honoraria, Other: steering committee membership ; Amgen: Honoraria, Other: steering committee membership ; Sanofi: Honoraria, Other: steering committee membership steering committee membership ; Novartis: Honoraria, Other: steering committee membership ; Roche: Honoraria; Gilead: Honoraria; Incyte: Honoraria, Other: steering committee membership ; Karyopharm: Honoraria. Decaux:Takeda: Honoraria; Janssen: Honoraria; Amgen: Honoraria; Celgene: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal